Calculations involving enthalpy
There are 2 ways of calculating enthalpies of reactions.
1) using bond energies (given on the left) as explained below
3) Using enthalpies of formation of reagents and products
The second and third methods are more accurate, because the bond energies are only an average, e.g., they depend on the molecular environment.
1) Calculating enthalpy of reaction based on bond energies
The energy transferred in a chemical process originates on the formation of bonds. There is also a cost; in order to promote a reaction some bonds must be broken, and that takes energy in. So, the enthalpy of reaction can be calculated by subtracting the energy cost (breaking bonds) from the energy released (making bonds). The reaction enthalpy calculator on the right will facilitate these calculations, which are shown below:
Let's start with a simple reaction: the combustion of methane (this is a calculation of enthalpy of combustion)
CH4 + 2 O2 --> CO2 + 2 H2O
Bonds broken:
4 C-H + 2 O=O
4*414 + 2*498 = 2652 (energy input)
Bonds formed:
2 C-O + 4 H-O
2*800 + 4*470 = 3480 (energy output)
Enthalpy of reaction => 2652 - 3480 = - 828 kJ/mol
The negative sign indicates that the reaction is exhotermic (releases energy in the form heat)
Plugging the numbers in the Reaction enthalpy calculator (on the right) provides the answer directly: -828 kJ/mol
(a more accurate method, using Hess's Law, gives -891 kJ/mol)
Another calculation of enthalpy of combustion: glucose
The structure of glucose is provided so that you can see what are the bonds that must be broken for the reaction to go.
C6H12O6 + 6 O2 --> 6 CO2 + 6 H2O
-Image-good-300.gif)
Answer: -2800 kJ/mol
That is pretty close to the experimental value of -2805.0 ± 1.3 (reference: http://webbook.nist.gov/cgi/cbook.cgi?ID=C492626&Mask=2 )
Another calculation of enthalpy of combustion: TNT
Notice that this is not a detonation, it is a combustion; if it was a detonation the extra oxygens needed to oxidize all the carbons in TNT wouldn't be able to get there in time, as lots of gases would be leaving fast (oxygen would have to be supplied via a solid substance, usually an oxidizing agent).
It is also interesting to calculate the volume of gas produced in this reaction. The production of large volumes of gases causes a substantial increase in the entropy of the system.This fact , allied to a high negative enthalpy, results in a very negative Gibb's energy, so that the reaction is very very favourable, as we know.
C7H2(NO2)3CH3 + 6.25 O2 --> 8 CO2 + 2.5 H2O + 1.5 N2
Below there are 2 representations of the TNT molecule:
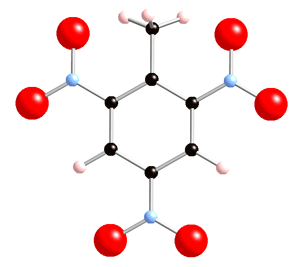
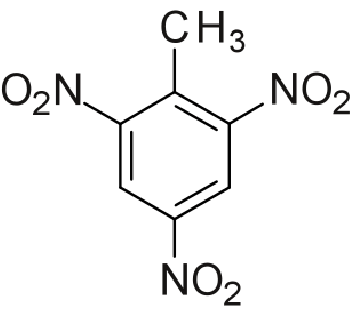
The bonds to be broken:
The bonding in NO2 presents resonance:
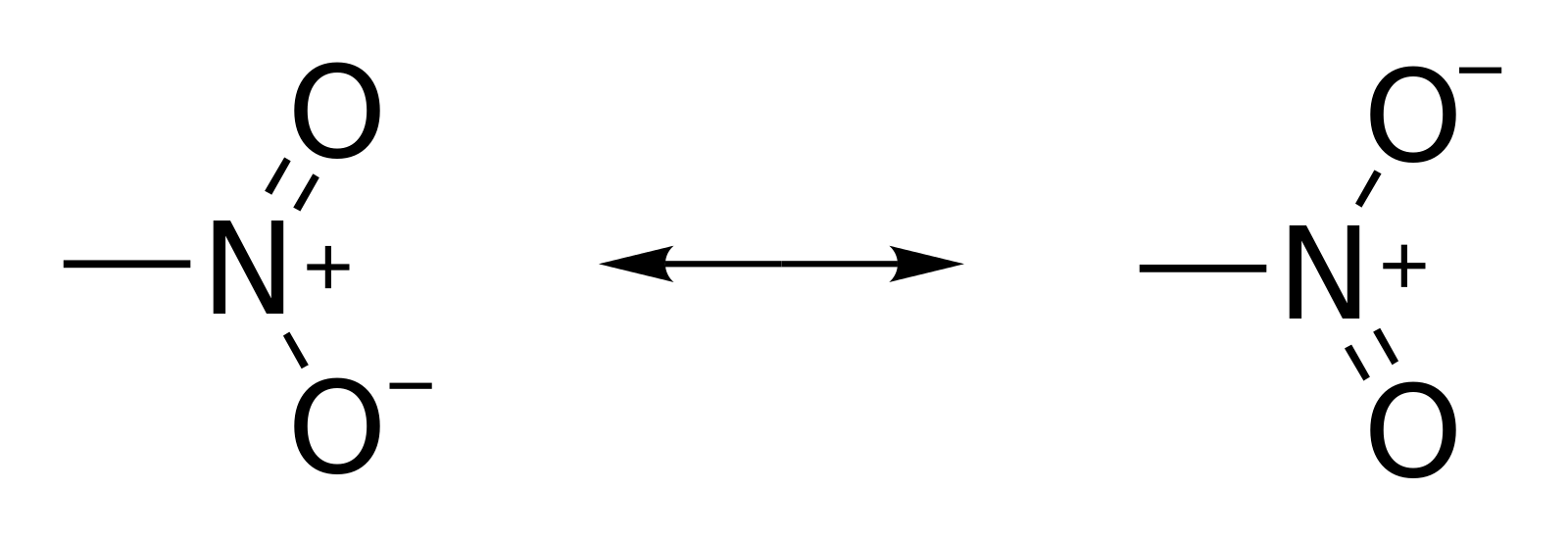
So, for each NO2 there are :
| group | bonds |
| N-O | 1 |
| N=O | 1 |
And also a C-N bond which is the attachment to the ring.
Total for 3 nitro groups is:
| group | bonds |
| N-O | 3 |
| N=O | 3 |
| C-N | 3 |
There is also resonance on the ring, so that there are 3 double bonds (C=C) and also 3 single bonds (C-C):
| group | bonds |
| C-C | 3 |
| C=C | 3 |
The methyl group presents:
| group | bonds |
| C-H | 4 |
plus one C-C bond which is the attachment to the ring.
The bonds formed are 16 C=O, 5 O-H and 1.5 N ≡ N
Now you can plug the numbers in the calculator on the right!
more calculations and examples on enthalpy>>
2) Calculate enthalpy of reaction using Hess's Law >>
