Igneous Rocks crystal structures
Quartz (there are various structures for quartz and the one below is only an example):
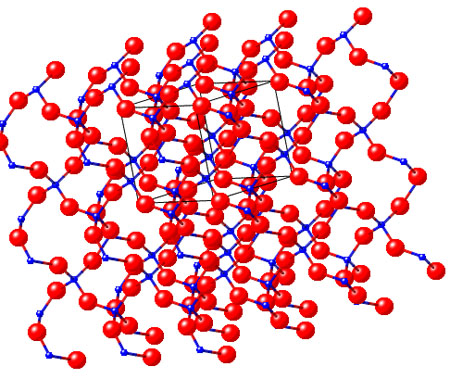
It can be seen that quartz is made of only 2 elements (or atom types): silicon and oxygen. Glass is also made of the same elements; the difference is that in the case of glass the atoms are not precisely arranged like in the image above. There is no crystal structure and glass is said to be amorphous. Glass is produced by heating sand. Sand is also made of Si and O and it is the result of rock weathering and erosion.
Feldspar
CaAl2Si2O8
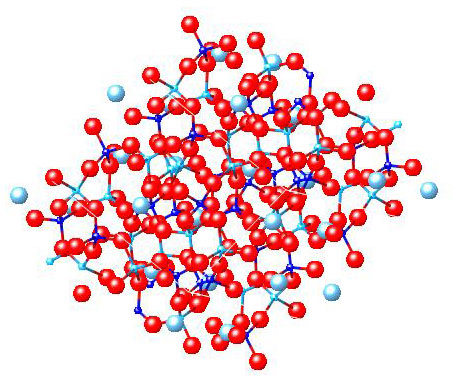
Feldspar also has a basic structure of Si and O but other elements also appear in the structure: these are Ca and Al.
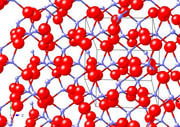
Olivine
Mg2SiO4
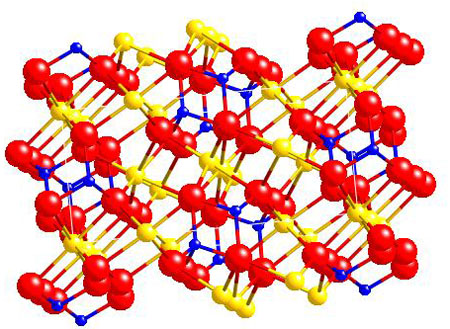
In the case of Olivine, atoms of Mg are present in the Si-O framework. It is interesting to note that the Mg atoms form octahedral structures (they ar at the centre). Likewise, the oxygen atoms are at the centre of tetrahedrals. The geometrical patterns produced by elements are very important to the characteristics of the materials . Each metal has its own typical geometries, which depend on factors like the oxidation state.