Ores - The rocks that are the source of metals
Most metals we use are not found in native form. They come from rocks.
These rocks, called ores, are submitted to a process called smelting, so that the metals are extracted. The metal atoms are usually bound to oxygen (oxides) or sulphur atoms (sulphides), among other elements.
For instance, gold often comes about bound to sulphur. Gold mines also produce a lot of sulphur. The gold mine close to BH (Brasil) has also a sulphuric acid plant to make use of the sulphur produced as a byproduct of the gold mining.
Below we see crystal structures of such rocks (ores):
Iron ores
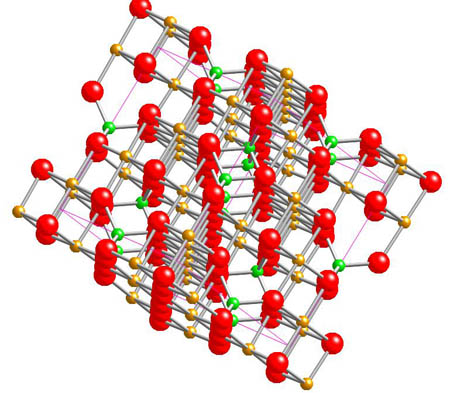
Magnetite - Fe3O4
The name comes from its magnetic properties!
Both golden and green atoms represent iron. The green
colour is used to show tetrahedral sites and golden represent octahedral sites. This interesting structure can be explored further on the animation.
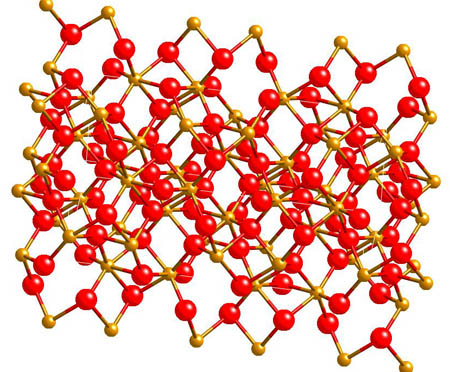
Hematite - Fe2O3
Copper ores

Cuprite - Cu2O
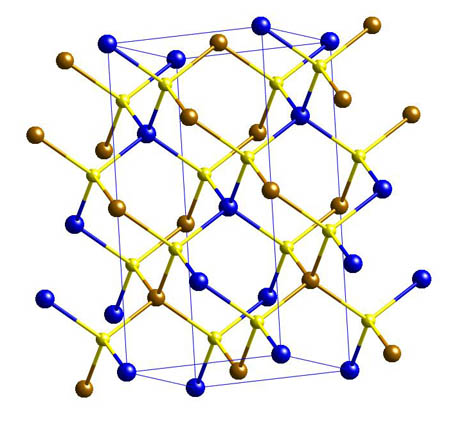
Chalcopyrite - CuFeS2
Mercury ore
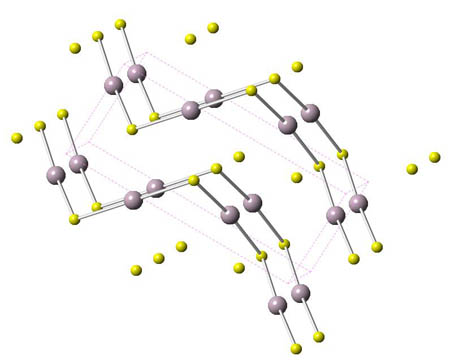
Cinnabar - HgS
Titanium ore
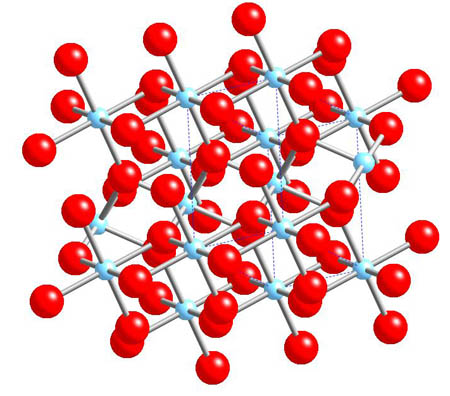
Rutile - TiO2
Aluminium ore
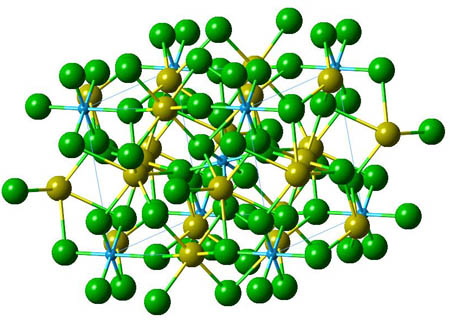
Cryolite - Na3AlF6
Cromium ore

Chromite - FeCr2O4
Cr atoms represented in pink.
This structure can be further explored in the animation.