::The Chemical bond::
Electronic subshells
Electrons are distributed in shells around the nucleus of the atom. The ground shell (the one with the lowest energy) is the one close to the nucleus. The oither shells have increasingly higher energy values. If an electron absorbs energy it will move to an outer shell. If it receives too much energy it can even move out of the atom (ionization). The fact electron are distributed in shells is a consequence of quantization of energy, so that there is not an energy value smaller than that of the separation between shells what implies that no electron can be in between shells.
These shells are sub divided in subshells, named s, p, d and f. Sub shells present 3D shapes that can be seen here.
The electronic capacities of subshells (s,p,d and f) are as follows:
s -2, p-6, d-10, f-14
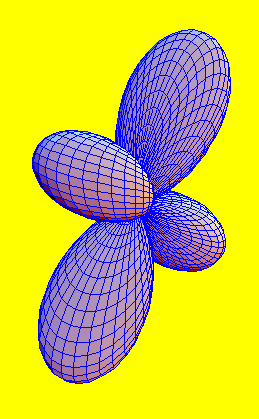
A d orbital
All numbers are even, as there is space for two sets of electrons, paired according to opposite spins (as required by Pauli exclusion principle).
Sub shells define the spatial distribution of electrons (orbitals). They have well defined shapes, some of which are seen on the left. These shapes are determined by solving the Schroedinger equation (gives quantum mechanical behaviour of electrons). It is like what Newton equations do for classical mechanics.
Elements that have valence electrons in s orbitals constitute the s-block, of the periodictable. The same definition applies to the p and d blocks. The d block consists of transition metals, where the distribution of electrons in the 5 different orbitals are responsible for the varied colours and geometries of the complexes made using these elements.
The question you normally get is, for instance, write the electronic configuration of aluminium.
Answer: The shells must be filled in the order of increasing energy: electrons go first to the lowest energy shell, and then start filling the upper levels. The diagram shown is normally used to find out this order:
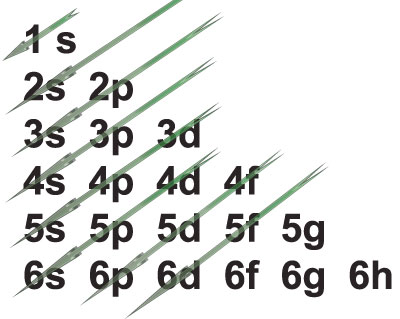
Electronic Sub shells in order of increasing energy
Aluminium has Z=13, so that it has 13 electrons. Filling the sub levels following the order given by the arrows above, and respecting the capacity of each sub level, leads to:
1s2 2s2 2p6 3s2 3p1
That shows also that the valence shell, n=3, has 3 electrons, 2 in a s type subshell and 1 ina p type subshell. The valence shell is the outer electron shell of an atom. It is very important because it determines the chemical traits of the element. The inside shells don't usually interact with the exterior (other atoms or molecules that approach).
If we consider the capacity of subshells s, p, d and f, t he diagram also shows us the capacities of the main shells. For instance, the 3rd shell has 3 types of subshell: s, p and d. That means that its total capacity is 2+6+10 = 18 electrons.
Capacity of atomic shells:
1st : 2
2nd: 8
3rd: 18
4th: 32
5th: 50
(outer shell: 8 electrons)
Other Example: write the electronic configuration of iodine (I).
Iodine has Z=53, so that it has 53 electrons:
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p5
That shows also that the valence shell of iodine, n=5, has 7 electrons.
Cl and Br also have 7 electrons in their valence shells, as discussed before. That is why I, Cl and Br are on the same group (column) of the periodic table!
Check out the atomic shell animations
© Ricardo Esplugas. All images in this site can be bought in an enlarged version. Please contact me on ricardochemistry@gmail.com

