Transition Metals
In the context of transition metal chemistry, the molecules are called complexes. They usually have on metal atom at the center, although in many cases more metal atoms can be present. The molecules that are bound to the metal centre are called ligands. The whole thing (metal + ligands) is called a complex, although it is also a molecule. The metal atom can be neutral or charged.
Metals can bond to a variety of ligands, like water, alcohol, acetone etc... Usually each metal presents a preference for a certain kind of ligand. Copper, for instance, "likes" to bind to nitrogen based ligands, like pyridine or the aminoacid histydine.
What makes transition metals very interesting are their d orbitals.
Important characteristics of transition metals include:
1) The have variable oxidation numbers. In Mn, for instance, it can vary from +1 to +7 (in the purple permanganate solution in water).
2) They are very coloured. By varying the oxidation number or changing the metal, a large variety of colours can be obtained (unlike compounds with alkaline metals, for instance, which are only white or grey). Vanadium complexes, for instance, can assume many colours, depending only on the oxidation number.
3) They are often used as catalysts.
4) They assume various well defined geometrical configurations.
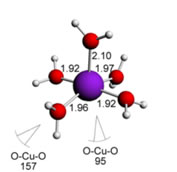
Some examples of complexes are shown below ( with the respective metal-ligand bond lengths and also bond angles) :
Cu(II) water complexes:
square planar structure
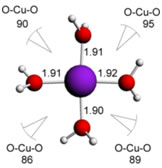
square based pyramid