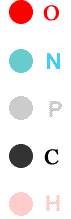Lipids (or fats)
Triglicerides and fatty acids
Are lipids, oils and fats the same thing? Yes. The word "oils" is normally used for lipids that are liquid at room temperature and "fats" for those that are solid. So, there is no difference between lipids, fats and oils. The majority of the fats are triglicerides, and that include everything we call fat in daily life.
Fats are made of fatty acids, which are are carboxylic acids with hydrocarbon chains
ranging from 4 to 36 carbons long.
Stearic acid, a saturated fatty acid
Stearic acid , shown above, has 18 carbons. It is saturated. That means that it is saturated with hydrogens. It takes the maximum possible number of hydrogens because there are no multiple bonds between the carbons.
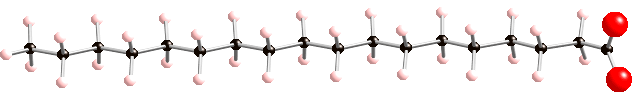
When a double bond is present, a molecule is called unsaturated (it has less hydrogens than it would without the double bond). An example of an unsaturated fatty acid is linoleic acid, shown below:
Linoleic acid, an unsaturated fatty acid
Linoleic acid has also 18 carbons (like stearic acid) but its structure is different because it has 2 double bonds. Each double bonds cause a kink on the structure. Because it has more than one double bond it is also called a polyunsaturated fatty acid.
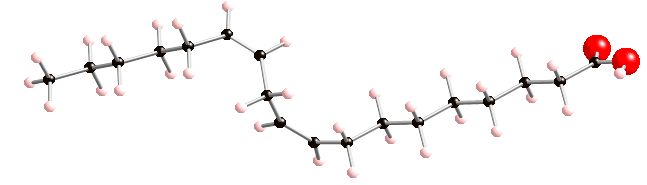
Arachidonic acid is another polyunsaturated fatty acid. It has 4 double bonds, and 4 kinks on the structure
Melting point of fatty acids
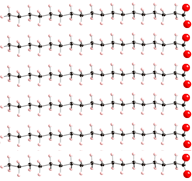
Saturated fatty acids are straight and as a result they can pack together closely:
Unsaturated ones are kinked, so that thir packing is more complicated:
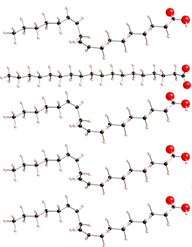
The result is that saturated fats have higher melting points, e.g., they can get solid more easily.
The simplest fat molecule consists of 3 fatty acids grouped together; this is called a triacylglycerol (or triglyceride).
A glicerol molecule (seen at the centre) provides the backbone which the fatty acids are attached to, by means of ester linkages:
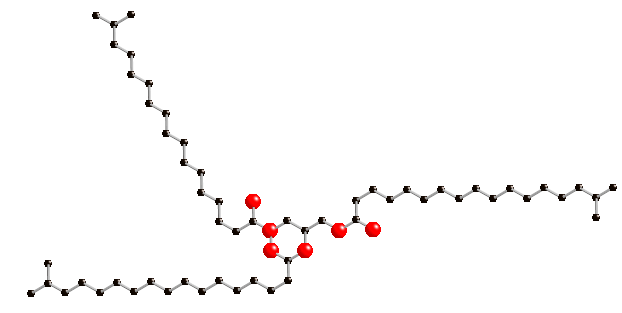
Triglicerides serve the purpose of storing energy in animals and plants. Even seeds have energy stored in triglicerides ( so that a growing seedling can get nourished), and that is one reason why the are such good food for animals. Most natural fats we find in food are triglicerides. Their long hydrocarbon chains (similar to those present in petroleum derived fuels like gasoline) are very exergonic upon oxidation, e.g. they release lots of energy. Fats are the food components with the highest energy density : 9 kcal / g (table below):
| Food component | Energy density | |
|---|---|---|
| kJ/g | kcal/g | |
| Fat | 37 | 9 |
| Ethanol (drinking alcohol) | 29 | 7 |
| Proteins | 17 | 4 |
| Carbohydrates | 17 | 4 |
In addition to serving as energy reserves, triglicerides serve as thermal insulation. Animals that live in cold weather ( seals, penguins, etc) rely on these molecules to keep warm.
The sperm whale finds yet another use for its triglicerides. It has about 4 tons of oil in the head, for the purpose of helping flotation and diving ( the oil gets denser as the temperature decreases because of diving. The physical properties of the woil are perfectly tuned to help with buoyancy at all depths, allowing the sperm whale to go fishing at the bottom of the sea.