Chemical Equilibrium
Exercises and problems
take the chemical equilibrium quiz (only 1 question to make you think)>>
1 - A mixture of 0,40 mol of sulfur dioxide and 0,20 mol of oxygen are heated in a 1 litre container in the presence of a catalyst, according to the chemical equation:
2 SO2 (g) + O2 (g) ⇌ 2 SO3 (g).
The concentrations of all 3 substances varied according to the graph:
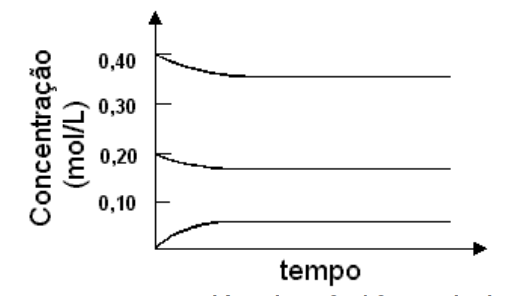
Ina second experiment, 0,40 mol of sulfur trioxide was inserted in a similar container and heated like the previous case, and using the same catalyst. The equilibrium concentrationm of SO3 would be:
a) 0,05 mol/L b) 0,18 mol/L c) 0,20 mol/L d) 0,35 mol/L e) 0,40 mol/L
Solution:
This question doesn´t require calculations - only a knowledge of the concept of equilibrium. More specifically, of the fact that the equilibrium concentrations are always the same (for a particular set of conditions) no matter the initial concentrations of the reactants.
As a result, the answer is that the equilibrium concentrations are the same as those in the example given. The desired concentration ( SO3 )can be read on the graph. This concentration was zero at the start of the first experiment so that it corresponds to the lowest line on the graph. The concentration is 0,5 mol/l.
Catalysts don´t affect the equilibrium, because they speed up both the direct and the reverse reaction equally.
2 - Brazil produces 6 x 106 of sulfuric acid annually using the contact process. One of the steps in the process, carried out at the pressure P and constant temperature, follows:
2 SO2 (g) + O2 (g)⇌ 2 SO3 (g) , KP = 4,0 x 104
At this temperature, what is the value of P for x2 SO3 / x2 SO2 x2 O2 to equal 6,0 x 104 ?
a) 1,5 b) 3,0 c) 15 d) 30 e) 50
x = molar fraction
KP = equilibrium constant
Solution:
-Aqui é exigido o conceito de fração molar e a fórmula para a constante de equilíbrio baseada em pressões parciais (KP).
For this question the concepts of molar fraction and partial pressures are needed.
KP , the equilibrium constant based on partial pressures, is similar to Kc , which is the equlibrium constant based on concentrations, so that:
KP = P2 SO3 / P2 SO2 P2 O2 = 4,0 x 104 (I)
The partial pressure of each gas in the mixture can be calculated because it is propotional to its molar fraction (x):
Pa = xa * P (II)
where Pa is the partial pressure and P is the total
pressure
- Substituting expression (II) in (I) :
x2 SO3 P2 / x2 SO2 P2 x2 O2P2 = 4,0 x 104
(x2 SO3 / x2 SO2 x2 O2) (1/P) = 4,0 x 104
4,0 x 104 * P =6,0 x 104 ==> P = 1,5
Answer: a)
3 - Consider the gas phase equilibrium:
CO(g) + H2O(g) ⇌ CO2(g) + H2(g)
where K, at 430 ºC, equals 4. In a 1,0 litre container, at 430ºC, 1,0 mol of CO, 1,0 mol of H2O, 3,0 mol of CO2and 3,0 mol of H2 where mixed and equilibrium was achieved.
a) In what direction will the reaction proceed until equilibrium is achieved?
b) Calculate the equilibrium concentrations of all substances involved.
Solution:
a) In order to find out the direction of the reaction, at this stage, we just calculate Q, the reaction quocient. Its has a formula similar to K, the only difference being that we plug in the concentrations at any point of the reaction. In the case we plug in the equilibrium concentrations, Q = K.
Plugging the concentrations given above, we get:
Q = 3*3/1*1 = 9.
This is larger than K (which is 4) , what means that the reactions is proceeding form right ro left (the more a reaction is shifted to the left, the lower its K).
The spreadsheet below calculates that:
b) The spreadsheet above shows equilibrium concentrations as functions of x. Plugging those in K and making it equal to its given value:
(3-x)2 / (x+1)2 = 22 ==> (3-x)/ (x+1)= 2 ==> 3-x = 2x+2 ==> -3x = -1 ==> x=1/3
So that equlibrium concentrations are:
[CO] = [H2O] = 4/3 mol / l
[CO2] = [H2] = 8/3 mol / l
4 - Cobalt can be obtained from its oxide by reduction using hydrogen or carbon monoxide. The following equations are given (at 550°C) :
I. CoO(s) + H2(g) ⇌Co(s) + H2O(g) K1 = 67
II. CoO(s) + CO(g) ⇌ Co(s) + CO2(g) K2 = 490
a) Calculate the constant K3 relative to the equilibrium:
CO(g) + H2O(g) ⇌ CO2(g) + H2(g)
at 550°C, based on K1 and K2
b) Reaction (I) above is exothermic at 550°C forming hydrogen. What would be the influence, in this reaction, of the use of :
- a catalyst
-an increase in temperature
-a pressure variation
Solution:
a) The procedure here is similar to that used in Hess's Law calculations, where chemical equations are manipulated just like in algebra. What is new here is that , when equations are added, K for the sum is the product of the individual K's of each reaction. Also, when a reaction is inverted, K becomes 1/K (in the case of enthalpy, as in Hess's Law, the enthalpy would become negative in this case).
Let's start: we need H2O(g) on the left side, so we invert reaction I and add it to II:
Co(s) + H2O(g) ⇌ CoO(s) + H2(g) K1-reverse = 1/67
CoO(s) + CO(g) ⇌ Co(s) + CO2(g) K2 = 490
-----------------------------------------------------------------
CO(g) + H2O(g) ⇌ CO2(g) + H2(g)
( CoO(s) and Co(s) were cancelled because they are in both sides)
K for the reaction obtained is given by the products of K's :
K = K1-reversa * K2 = 490/67 = 7,3
b) According to the Le Chatelier Principle, a reaction in equilibrium will adjust to minimize the change in conditions.
Because the reaction is exhotermic, an increase in temperature can be minimized by moving the equilibrium to the opposite direction (in this case an endothermic reaction) , in order to absorb the extra heat.
A catalyst never affect an equilibrium.
A pressure change will not affect the equilibrium because there are the same number 0f mols of gases in both sides of the reaction.
take the chemical equilibrium quiz (only 1 question to make you think)>>

