Pure forms of carbon
Carbon is everywhere. Along with other elements carbon forms all sorts of things: rocks, plants, animals (proteins, DNA, lipids, sugars,etc... ), plastics, etc.
There are some materials, however, that contain only carbon. These are pure forms of carbon .
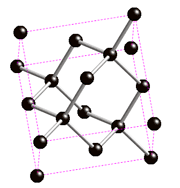
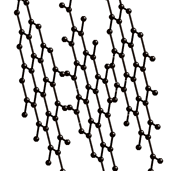
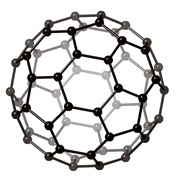
They are called allotropes, because they only differ by the geometrical arrangement of the atoms , only carbon atoms, as seen as black spheres on the images below.
It is interesting to notice how crystal structure can change drastically the properties of materials, even when they have the same chemical composition!
All these carbon materials can be 100% pure, but usually they have tiny amounts of impurties.
Diamond and graphite are traditionally the main allotropes of carbon, as fullerenes and nanotubes were discovered more recently. Because the carbon atoms in diamond are more tightly bound than in graphite (as it is the hardest material known), it is more difficult for impurities to get into the diamond lattice and as a result diamonds are often more pure than graphite.
A 100% pure diamond would be totally transparent. When they have impurities they are often coloured! Most commonly they are coloured blue or yellow.
COAL
What about the most seen form of carbon: coal? Coal is not a crystalline material. It is amorphous. That means that their atoms are not arranged in perfectly regular geometrical strucutres, as seen on the images above.
There are various kinds of coal, some of them containing lots of impurities. A common impurities is sulfur (S) and that causes acid rain (sulfuric acid) as powerstations burn lots of it.
The purest form of coal is called Anthracite. It can have up to 98% carbon and it is the most valuable coal. Unfortunately it is hard to find.
(anthracite, the purest coal, is only 92 to 98% pure carbon)
© Ricardo Esplugas.