Electrochemistry
REDOX Reactions
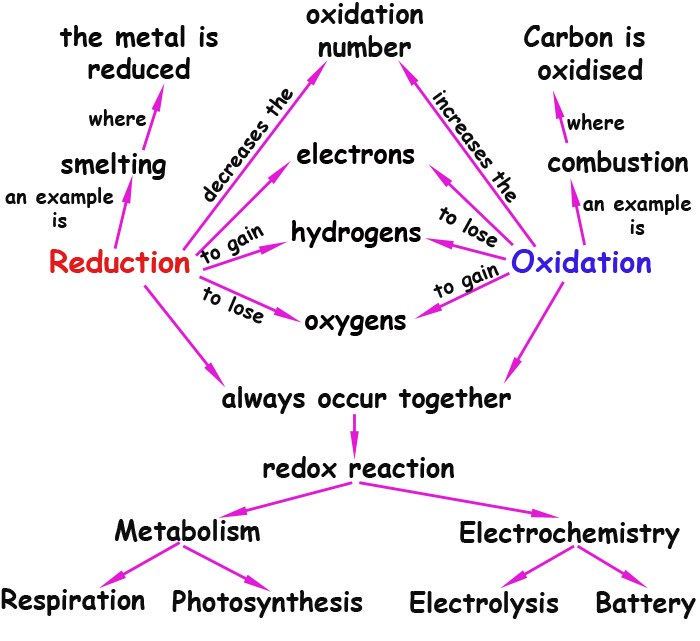
This map displays various informations about REDOX reactions. I would like to call your attention to some :
1) Oxidation and reduction always occur together . Oxidation means to lose (or to give) an electron so that this electron lost must go anywhere . It won't disappear. That means that some other substance around will have to receive that electron and as a result it will suffer a Reduction.
In the study of the table of reduction potentials, we see half reactions which deal only with the reduction part of the REDOX reaction. It is done on this way to make it simpler but it is important to keep in mind that this reduction (half-reaction) would not occur without the oxidation.
2) It is important to observe that oxidations are not necessarily a gain of oxygens, it may be a loss of electrons. That means that oxidations can occur in reactions without oxygen !
3) REDOX reactions arethe essence of fundamental processes like respiration and photosynthesis, without which no life would be possible in this planet. In these complicated metabolic pathways, the electrons are transported by various enzymes and carried across membranes to various receptors along the way. In the case of respiration the electrons end up in the oxygen (from the air we breath in) so that it is reduced to water, which is expelled as a waste product.
