Reduction Potential
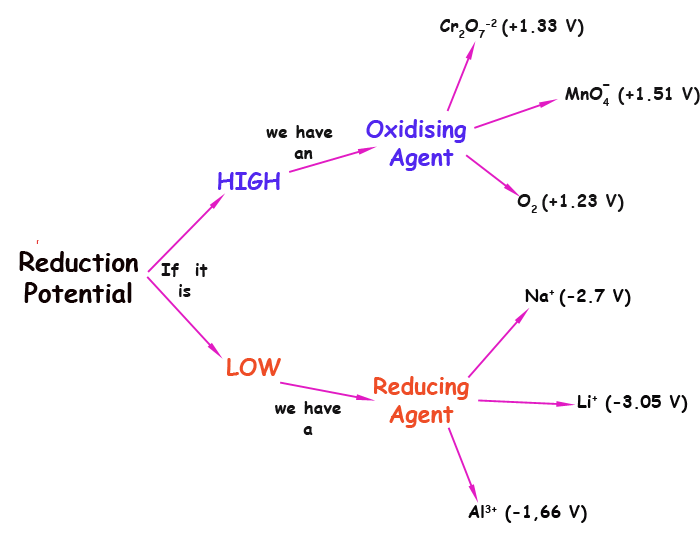
The reduction potential corresponds to a half-reaction, which is only the reduction part of the oxi-reduction reaction. This half reaction cannot occur as such; it is needed the oxidation. As we have seen, the REDOX process includes reduction and oxidation, and we cannot have any of the two parts separately, without the other. However, it is useful to think of the reduction half reaction, to assign reduction potentials to substances.
Examples of half-reactions -
a)The reduction of Na+ :
Na+ (aq) + e- --> Na (s) ; Eo = -2.7 V
Redox potentials are given in volts (V). A negative value, as above, means that the reaction is not favourable. In this case, not favourable means that Na+ doesn't want to receive any electrons. In fact, it would like the opposite: to donate electrons. Because of its very low value (-2.7 V) it could even force the electrons into another substance; the other substance would be as a result be reduced. This means that substances with very low reduction potential are good as reducing agents. They reduce other substances, while they get oxidised themselves.
In fact that is what sodium does. It can reduce even water, producing hydrogen and hydroxyl ions . The hydroxyl ions make the water alkaline, and that is why sodium is an alkaline metal!
b) The reduction of MnO4 -
MnO4 - (aq) + 8 H+ (aq) + 5e- --> Mn2+ (aq)+ 4 H2O (l); Eo = +1.51 V
In this case the reduction potential is negative so that the half reaction is favourable. That mens that the permanganate ion wants to receive electrons. Because of the relatively high value of the potantial in this case, this ion has the power to pull electrons from various substances. It is an oxidising agent. It likes to get reduces, and for that it must pull the electron from elsewhere.
Continuation - the Galvanic Cell >>
