Electrostatic Force
Electromagnetic forces, gravitational forces and nuclear forces are all the forces we have on the Universe!
The electrostatic force is part of the more general electromagnetic force, that includes magnetism.
The gravitational interaction, or weight, is the result of the interaction of a mass with a gravitational field (produced by another mass(ess)). Likewise, the electrostatic force is the result of an interaction between a charge with the electric field produced by another charge(s).
::Calculating the force::
The electrostatic force between 2 charged particles only depends on the intensity of the charges and the distance between them:
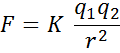
where, q1 and q2 are the charge values, r is th distance between them and K is a constant.
K = 9 * 109 N m2 / C2
This is also called Coulomb's Law.
Note: If there are more than 2 charges, you consider the total force based on the forces beteen 2 charges at a time. In other words, the interaction takes place in pairs. In the examples below that will become more clear.
::Examples of calculations ::
1) What is the repulsive force between 2 protons in the nucleus of an atom, where they are only 5.0 x 10-15 m apart?
The charge of the proton is 1.6 x 10-19 C
It is important ot make sure that all units used are the same as the ones in the constant K, namely, m, C and N.
F = (1.6 x 10-19)2 * 9 * 109 / (5.0 x 10-15 )2 = 9.2 N
That is approximately the weith of a 1 kg object!! It is an incredibly huge force for such microscopic particles to exert! No wonder the nuclear force is so enormous, in order to keep the nucleus together!!
exercises for electrostatics >>
::The Electric Field::
The electric field can only be detected by means of a charge. If we place a charge somewhere in space and there is a force on it, that means that an electric field is present.
The electric field is given by:
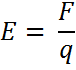
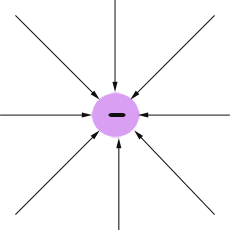
The electric field is often represented by lines.
__________________________________________
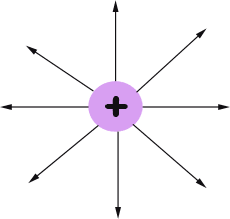
Field lines go into negative charges and out of positive charges.


