Graphite
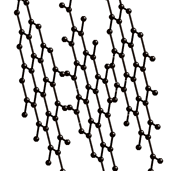
Graphite consists of a layered structure, made of hexagonal rings of carbon (like benzene). Each layer is like chicken wire. Its name comes from the greek: graphos--> write. It has long been used in pencils because its layers are losely bound so that they can be laid onto paper just by rubbing. For the same reason graphite is also used as a lubricant.

Layers of graphite (blue colours used for artistic purposes)
The bonds between the carbon atoms are present only in the planes, as seen on the image above, so that there is no inter plane bonding. That is why graphite is quite soft and it is used as a lubricant.
Another interesting structure that is made of pure carbon is diamond. In this case, there is a three dimensional bonding instead of this layered structure. That is why diamond is not used for writing; in fact , it is the hardest material on earth.
Graphite is also used in the nuclear industry. It is placed inside the core of nuclear reactors like for onstance AGR's (advanced gas reactors).
Graphene is an extremely strong material, which consists of a single layer of graphite!
Other images of the graphite structure:
© Ricardo Esplugas.