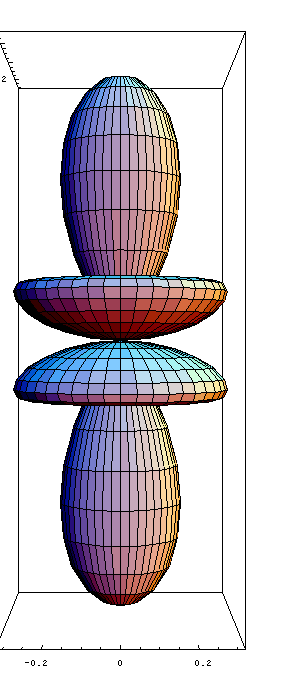
Molecular Orbitals
They represent the spatial distribution of the electrons around the atoms forming a molecule. Because the electrons are responsible for chemical bonding, it is very interesting to know their distributions.
Example:
Benzene orbitals
The most important orbitals of a molecule are its frontier orbitals, because they govern its chemistry.
Here the HOMO (highest occupied molecular orbital) and LUMO (lowest occupied molecular orbital) of benzene are pictured. If you don't remember what benzene looks like, it is shown at the bottom of the page, vibrating!
(these were calculated by Ricardo using the ADF - amsterdam density functional - software) .
Other calculated results are also shown: the electron density distribution and also one of its vibrational modes.
Other important structure made of carbon is graphite, which is also hexagonal.
HOMO
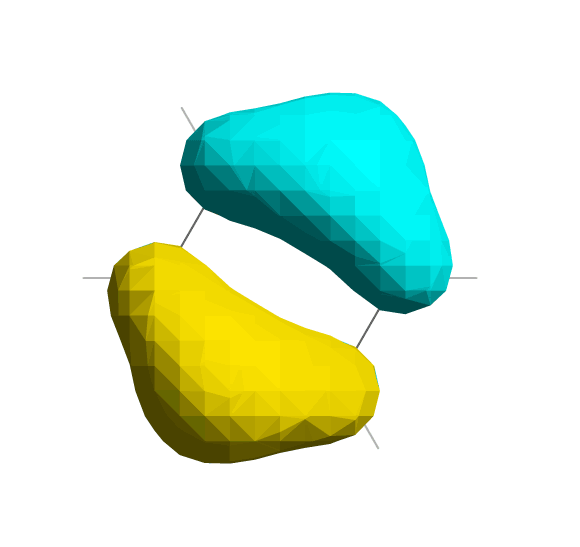
LUMO
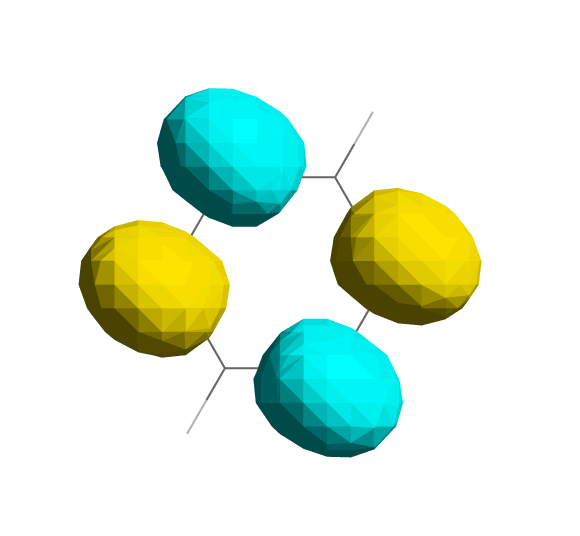
Electron density:
(this is not an orbital)

Benzene vibration
© Ricardo Esplugas. All images in this site can be bought in an enlarged version. Please contact me on ricardochemistry@gmail.com