The Chemical bond
IONIC BONDS
Ionic bonds consist of the mutual attraction between ions. They must have opposite charges, obviously, so that they can attract each other.
For instance, in the NaCl crystal (shown below), the positive Na+ attracts the negative Cl- :
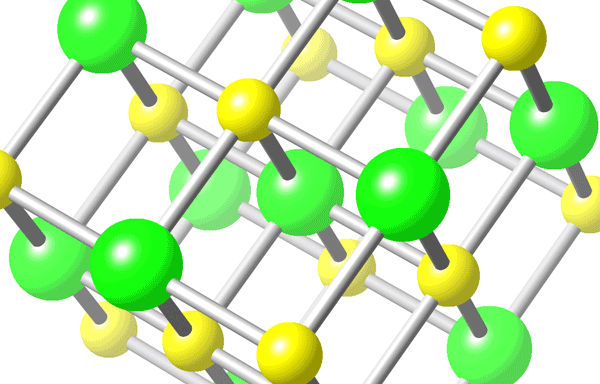
The larger ions (shown in green) are the Cl- and the smaller ones (yellow) are Na+. Anions are usually larger because of the extra electron.
These ionic bonds form when Na and Cl get close together, so that one electron from Na is transferred to Cl spontaneously, forming the ions and as a result the chemical bond. This type of chemical bond consists of an electrostatic attraction.
Every ion in the crystal is interacting with all its neighbours (repeling the ones with like charges and attracting the ones with opposite charges). That means that it is not only a bond between 2 ions , but a more complex situation.
The ionic bond are very strong so that it makes compounds that are usually solids with a high melting point.
Ionic bonds are usually formed between elements of groups 1 and 2 of the periodic table, which form cations (positive ions) and elements from groups 6 and 7, which form anions (negative ions).
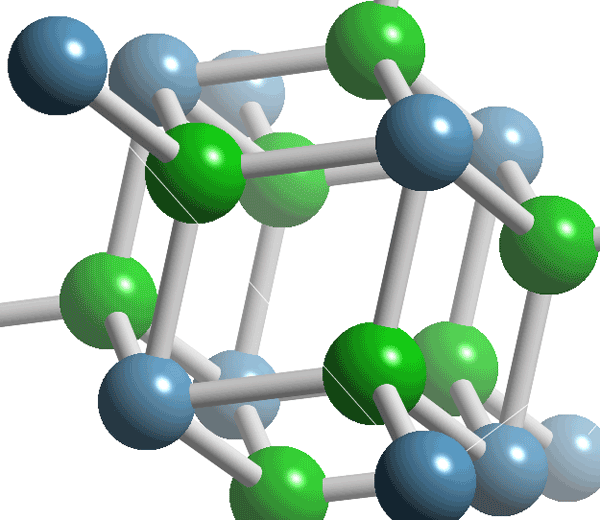
Other examples: CaF2 (below):
© Ricardo Esplugas. All images in this site can be bought in an enlarged version. Please contact me on ricardochemistry@gmail.com

