The Chemical bond
COVALENT BONDS
Covalent bonds are made between atoms that share their electrons. The result is the formation of molecules. The atoms don't need to be ionised in order to stick to the others.
A molecules of sucrose is shown below:
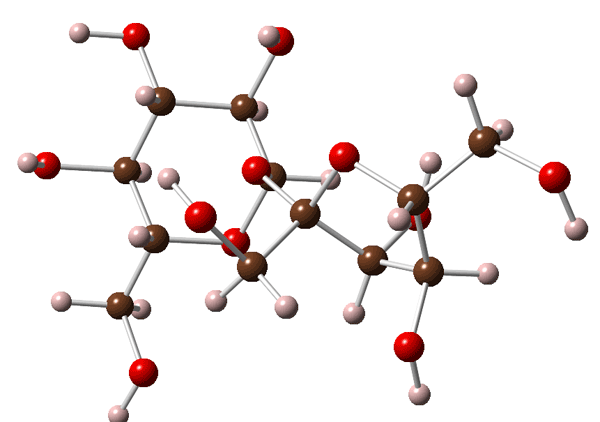
Sucrose is a sugar (the termination -ose is present in all sugars), so that it is formed of carbon, hydrogen and oxygen (carbohydrate).
© Ricardo Esplugas. All images in this site can be bought in an enlarged version. Please contact me on ricardochemistry@gmail.com

