Ligands
What is the difference between molecules and complexes?
Transition metal complexes are in fact molecules. There is no difference. Molecules are usually called complexes in the context of transition metal chemistry.
A transition metal complex consists of a metal atom (usually one only, but more metal atoms are possible) and one or more ligands attached to it. The ligands can be various molecules, like water, CO, CO2, ethanol, methanol, acetone, etc...
The type of ligand chosen will affect the geometry and the reactivity of the complex as a whole.
Here some complexes with different types of ligands are shown (with the respective bond angles and bond lengths):
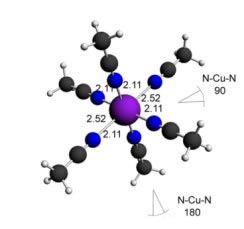
Silver (II) acetonitrile complex-6 ligands - Octahedral
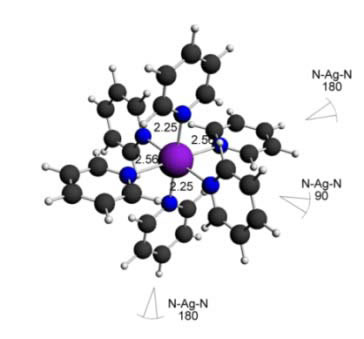
Silver(II) pyridine complex - 6 ligands - Octahedral

Silver(II) acetone complex - 4 ligands
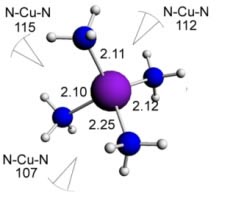
Cooper (I) ammonia - 4 ligands - Tetrahedral
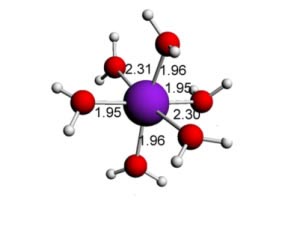
Copper (II) water - 6 ligands - Octahedral (distorted)
