Chemical Equilibrium
Exercises and problems
take the chemical equilibrium quiz (only 1 question to make you think)>>
1 - A mixture of 0,40 mol of sulfur dioxide and 0,20 mol of oxygen are heated in a 1 litre container in the presence of a catalyst, according to the chemical equation:
2 SO2 (g) + O2 (g) ⇌ 2 SO3 (g).
The concentrations of all 3 substances varied according to the graph:
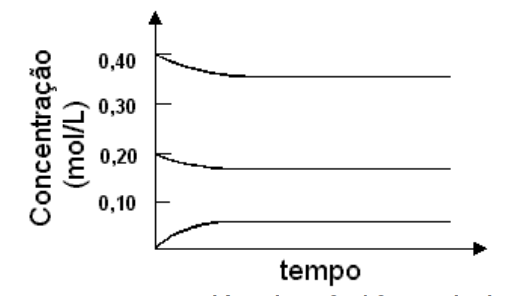
Ina second experiment, 0,40 mol of sulfur trioxide was inserted in a similar container and heated like the previous case, and using the same catalyst. The equilibrium concentrationm of SO3 would be:
a) 0,05 mol/L b) 0,18 mol/L c) 0,20 mol/L d) 0,35 mol/L e) 0,40 mol/L
2 - Brazil produces 6 x 106 of sulfuric acid annually using the contact process. One of the steps in the process, carried out at the pressure P and constant temperature, follows:
2 SO2 (g) + O2 (g)⇌ 2 SO3 (g) , KP = 4,0 x 104
At this temperature, what is the value of P for x2 SO3 / x2 SO2 x2 O2 to equal 6,0 x 104 ?
a) 1,5 b) 3,0 c) 15 d) 30 e) 50
x = molar fraction
KP = equilibrium constant
3 - Consider the gas phase equilibrium:
CO(g) + H2O(g) ⇌ CO2(g) + H2(g)
where K, at 430 ºC, equals 4. In a 1,0 litre container, at 430ºC, 1,0 mol of CO, 1,0 mol of H2O, 3,0 mol of CO2and 3,0 mol of H2 where mixed and equilibrium was achieved.
a) In what directionwill the reaction proceed until equilibrium is achieved?
b) Calculate the equilibrium concentrations of all substances involved.
4 - Cobalt can be obtained from its oxide by reduction using hydrogen or carbon monoxide. The following equations are given (at 550°C) :
I. CoO(s) + H2(g) ⇌Co(s) + H2O(g) K1 = 67
II. CoO(s) + CO(g) ⇌ Co(s) + CO2(g) K2 = 490
a) Calculate the constant K3 relative to the equilibrium:
CO(g) + H2O(g) ⇌ CO2(g) + H2(g)
at 550°C, based on K1 and K2
b) Reaction (I) above is exothermic at 550°C forming hydrogen. What would be the influence, in this reaction, of the use of :
- a catalyst
-an increase in temperature
-a pressure variation
take the chemical equilibrium quiz (only 1 question to make you think)>>

