Chemical Equilibrium
Introduction
take the chemical equilibrium quiz (only 1 question to make you think)>>
more examples and solved exercises>>
One very interesting question is: can a chemical reaction go backwards? Certain reactions we commonly see don´t seem to be able to return to its initial state, like combustions or explosions for instance. But the answer to this question is also very interesting: reactions can go forward and backwards at the same time! This is what we know by chemical equilibrium. The double arrow (below) represents that:
Example: A reaction between two gases:
N204 ⇌ 2NO2
This is a very interesting reaction because it can be followed visually. N204 is colourless and NO2 is red-brown. It is possible to force the equlibrium to shift one way or the other by varying the temperature (Le Châtelier´s Principle-more on that below), and them we see the red-brown colour disappering and reappearing (there are lots of youtube videos that show that, for instance look at that one).
So, imagine that we start the reaction (at 25 °C) by inserting 2 mol of N204 in a 1 l reaction vessel (initially empty). After equilbrium is achieved, there will be 0.1 mol of NO2 and 1.95 mol of N204 . If instead, we insert 4 mol of NO2, the result is exactly the same. Those are the equilibrium concentrations for this reaction (at this temperature) and it will be achieved no matter it starts with NO2 or N204 (these calculations were realized using an equilibrium constant of 4,6 x 10-3 at 25 °C).
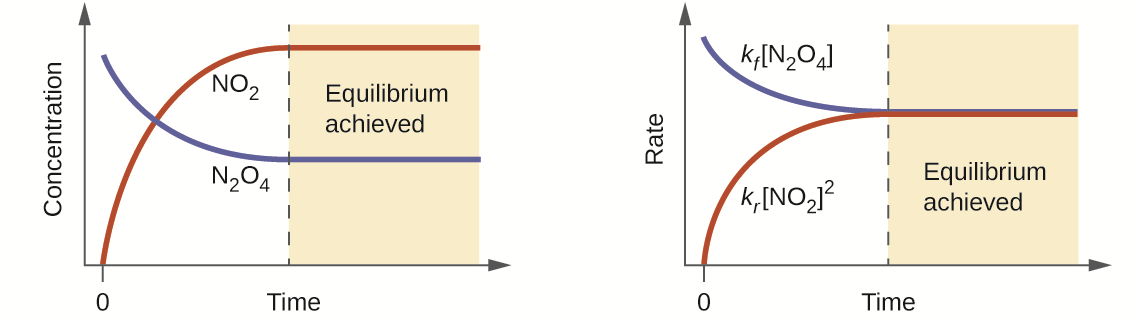
Equilibrium is achieved when the rate (or velocity) of the forward reaction equals that of the reverse reaction.
Rates are given by the following expressions (where k, the rate constant, depends on the particular reaction) :
Forward reaction:
N204 → 2NO2
rate = kf [N204]
Reverse reaction:
2NO2 → N204
rate = kr [NO2]2
OBS: Notice that NO2is squared because its stoichiometric coefficient is 2. The same applies for N204 and everything else you may encounter.
The equilibrium constant - Kc
At equilibrium the rates must be equal:
kf [N204] = kr [NO2]2
kf / kr = [NO2]2 / [N204] = Kc (equilibrium constant)
Kc is the equilibrium constante based on the concentrations of the reactants.
Kc for the reverse reaction is the inverse of Kc for the direct reaction.
For reactions involving gases it may be more convenient to do calculations using partial pressures, rather than concentrations:
The equilibrium constant - Kp
Using the same example above,
Kp = P2 NO2 / P N2O4
Onde P N2O4 is the partial pressure of N204 and PNO2 is the partial pressure of NO2 . Both add up to the total pressure in the system.
Example: If atmospheric pressure is 1 atm and oxygen is 20% of the atmosphere, P O2 = 0,2 atm.
The partial pressure can be calculated from the molar fraction (x) .
Pa = xa * P
The mole fraction is how many moles of a given substance are present in the system.
Example: In a mixture of gases there are 3 moles of nitrogen , 4 moles of argon, 2 moles of oxygen and 6 moles of hydrogen, what is the molar fraction of nitrogen?
The total number of moles is 15. The mole fraction of nitrogen is 3/15.
If the pressure of the mixture of gases is 10 atm, the partial pressure of nitrogen is 10 * 3/15 = 2 atm.
The reaction quocient (Qc)
Qc is based on concentrations and is similar to Kc. It is useful to tell in what direction an equilibrium reaction is going.
The expression for Kc involves the concentrations at equilibrium. In the case of Qcthose are usually the initial concentrations, before the equilibrium is achieved. Qcwill be different from Kc ; if it is higher, the concentrations of products are higher and those of reactants are lower than those at equilibrium, so that the reaction will proceed to the left in order to achieve equilibrium, and vice-versa.
Em resumo:
Qc < Kc
reaction proceeds from right to left until equilibrium is achieved
Qc >Kc
reaction proceeds from left to right until equilibrium is achieved
Concentrations of solids and pure liquids
Solids and pure liquids are ignored from the expression for K.
Example:
CoO(s) + H2(g) ⇌ Co(s) + H2O(g)
Kc = [H2O] / [H2]
Cobalt and cobalt oxide are solids and therefore excluded from the expression for Kc .
Add equations / multiply K's
If two or more reactions are added, K for the resulting reaction is given by the product of the K's of the reactions added.
So, reactions can be rearranged and added together like we do in algebra. This is also done in the context of Hess Law, to calculate the enthalpy of one reaction based on the enthalpies of others that are added together .
- Important points:
- The equilibrium is dynamical : Although the concentrations of each substance remain constant, the reactions are at work, e.g., products and reagents are forming all the time. The constant concentrations are just the net result !
- A catalyst doesn´t change the equilibrium, because it accelerates both the direct and the reverse reaction, to the same extent. The equilibrium constant remains the same if a catalyst is added. The difference is that the reactions will be accelerated so that equilibrium is reached quicker.
- The substances involved have constant concentrations but it doesn´t mean that they are all the same. The proportion between amount of products and reagents at equilibrium is given by the equilibrium constant, which may depend on various factors, including temperature and pressure. Le Chatelier's principle describes these changes (below).
Le Chatelier's principle
A concise definition of this principle (Gall, John (2002). The Systems Bible (3rd ed.). General Systemantics Press) is as follows:
When a settled system is disturbed, it will adjust to diminish the change that has been made to it.
The idea is somehow related to the concept of homeostasis in biological systems, which comprises negtive feedback systems to mantain variables like body temperature and concentrations of various substances in the blood, for instance, stable.
It is a qualitative (and not quantitative effect) so that no calculations are involved in this topic. You normally get questions on to which side will the equilibrium shift depending on the change in conditions.
There are 3 conditions that can be changed:
HEAT
If the settled system is an exothermic reaction (A-->B) in equilibrium, and the change is an input of heat, the adjustment will be a shift towards the endothermic reaction (B-->A), which is the reverse reaction. This way, heat is removed by the endothermic reaction and the change(which is the heating of the system) is diminished.
PRESSURE
If the volume of reagents is different from the volume of products, the equilibrium is shifted if there is a change in pressure. An increase in pressure causes an adjustment to the system that is shift in the equiibrium towards the side whose volume is smaller, do that the change is diminished, and vice-versa.
Por example, in the following reaction (Haber porcess for the porduction of ammonia):
N2 (g ) + 3H2 (g ) ⇌ 2 NH3(g )
There are 4 moles of gases on the left and 2 on the right. An increase in pressure will shift this equilibrium to the left so that the change is diminished, with less molecules of gas in the reactor vessel.
CONCENTRATION
If the change to the settled system is an increase in the concentration of some reactant, the adjustment is to consume this reagent in excess. Using the previous example again (the Haber process), we see that an increase in concentration of amônia moves the equilibrium to the left (to consume the extra ammonia).
In a production plant, however, the opposite is required. Ammonia is the desired product. So, by removing ammonia as it is produced, the equilibrium is shifted to the right and more ammonia is produced. This strategy is used in chemical plants.
Calculations involving Kc :
- Calculate Kc given the concentrations of all the substances involved. This is very easy, you just have to plug the numbers in the formula.
-Predict the direction of a reaction given the equilibrium constant and the concentrations of reactants and products. In this case you need to calculate Qc and Kc and compare their values.Example.
-Calculate equilibrium concentrations given Kc and all but one equilibrium concentration. Easy, you just need to plug the values in the expression
-Calculate equilibrium concentrations, given the equilibrium constant and the initial concentrations. For that you need to use some algebra as in this example.
- Calculate Kc for a reaction based on the Kc's of other reactions that are added together, in order to obtain the reaction asked.
-Calculate Kc based on a graph
more examples and solved exercises>>
take the chemical equilibrium quiz (only 1 question to make you think)>>

