Nuclear reactors-Intro
A nuclear power plant resembles an engine as its purpose is to convert heat energy, which comes from fuel consumption, into kinetic energy (movement) which, in the case of the power plant, will be converted into electric energy. The nuclear reactor produces steam which powers turbines that are usually in a separate building. The turbines will drive electric generators. Likewise, all thermoelectric plants use fuel to generate heat, produce steam and drive turbines and generators. The difference here is that heat is produced by nuclear reactions and not by burning fossil fuels (like gas, oil or coal).
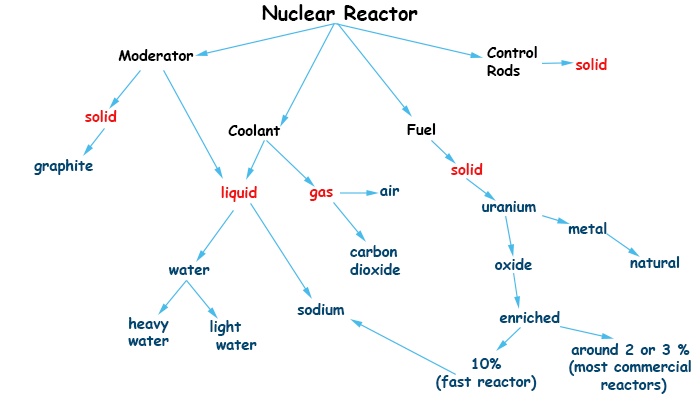
The concept map below show the main components of a nuclear reactor. Like any engine, it uses fuel, coolant and it also must have a control mechanism. It has more in common with the jet engine, because it works better at constant full power, and less in common with Diesel engines that can have its power output constantly changed. This fact will be recalled when describing the Chernobyl accident.
The only basic component that is unique to the nuclear reactor is the moderator, which will be discussed in detail.
NUCLEAR FISSION
Uranium is one of the largest atoms that exist in the universe. It has 92 protons and 143 neutrons (in the case of U-235). Because it has so many protons very close together, that are positively charged, there is a lot of electrostatic repulsion in its nucleus, and as a result it is relatively unstable.
Because of its instability, it will break apart if hit by a neutron. Because a neutron has no electric charge, it can fly right in the centre of the nucleus without suffering any deflection. This process is called nuclear fission. The fragments of the fissioned uranium atom (fission products) will move apart at very high speeds and as a result it will heat up the medium.
Most interesting about nuclear fission is the fact that it can go on as a chain reaction. Each fission yields 2 or 3 neutrons in addition to the nuclear fragments. These neutrons produced can hit other uranium atoms and produce new fissions so that the process keeps going until all the uranium atoms are finished (all the fuel is consumed). That is the essence of the nuclear reactor.
There is a few technical details that must be considered in order to initiate and maintain the nuclear fission chain reaction:
1) If the neutrons travel too fast, it is unlikely that they will ever hit an uranium atom. They must have their velocity reduced.
2) Uranium extracted from mines have about 99.3% U-238 and 0.7% of U-235. The latter is the isotope that is easily fissionable and is useful as a fuel in nuclear reactors.
NUCLEAR FUELS – NATURAL URANIUM AND ENRICHED URANIUM
U-235 is the fissile isotope. U-238, which is predominant, is not fissile but it is fertile. That means that it can receive one neutron, during its stay at the nuclear reactor, and become U-239 which decays into Pu-239 (plutonium), an element that is fissile and can be used as a fuel in nuclear reactors and also in atomic bombs. It is very difficult to remove the produced plutonium from the highly radioactive mix of elements that constitute spent nuclear fuel, and only very few countries can do it in an industrial basis (nuclear fuel reprocessing) .
U-235 is the fissile isotope so that it is interesting to increase its amount. Its percentage can increase from 0.7% to around 2 or 3% to be used as a fuel in most reactors nowadays. This process is called uranium enriching. The percentage can be much higher if the purpose is to build a bomb. Uranium enriching is also a tricky and very expensive process. It will be explained in more detail.
The first nuclear reactors exploited the fertility of U-238 and were used to produce plutonium for bombs. The Windscale piles, which started operations in the north of England in 1957, were that kind of reactor. They were responsible for the worst nuclear disaster in the world up to that time. They used natural uranium as a fuel and graphite as the moderator.
MODERATORS
Those are materials used to moderate the speeds of the neutrons inside the reactor, e.g., to reduce their speeds. Neutrons collide with the atoms in the material, which absorb some of the speed. This process is more efficient when the mass of the atoms used for this collisions are similar to those of the neutrons. Hence, a good substance for a moderator is water, because it has hydrogen atoms. Another material that is used a lot is carbon, in the form of graphite.
The speed of the neutrons must be reduced to increase its probability of hitting an uranium atom and maintain the chain reaction.
A moderator is not needed in the case of highly enriched uranium, because in this case there are plenty of U-235 atoms and is unlikely to miss it. That is the case in fast reactors and bombs.
CONTROL RODS
Those are rods that are inserted into the reactor core to control its level of power or to shut it down. The action of inserting the rods all the way to shut down the reactor is called a scram.
Control rods are made of a material that absorbs neutrons like cadmium or boron. If the neutron flux is interrupted, the chain reactions stops.
COOLANT
This is the material that will remove heat from the reaction core and transport it to the steam generators and turbines in order to produce electricity. It is typically water, but not always.
The lack of coolant would cause an overheating of the reactor, what could lead to a nuclear disaster. A ruptured pipe, for instance, could cause a LOCA (Loss of Coolant Accident).
::::::::::::::: Concepts related to accidents::::::::::::::::
Fission products
These are the products of the fission of the uranium atom. Because they contain excess neutrons, they are radioactive and very dangerous. They stay inside the reactor and are removed along with the spent fuel for long term storage. If something goes wrong they may be released on the environment and cause disease and death.
Steam explosion
If a reactor gets out of control and the heat inside increases too much, beyond the operating range, the water will start to boil at an extremely high pressure and the reactor vessel may explode. That is what happened at Chernobyl.
Decay heat
It is the heat produced by the radioactive decay of fission products, and it is responsible for a small fraction of the total amount of heat produced in the reactor. Because of decay heat, reactors must be cooled even after shutdown to avoid a meltdown. It takes about 2 hours for the decay heat to get significantly reduced. Decay heat caused the accidents of TMI and Fukushima.
Spent fuel pool
It is the pool of water where the spent fuel is deposited, right after removal from the reactor, so that its radioactivity can decrease before it can be sent away for reprocessing or disposal. The pool must be cooled because the fuel produces decay heat.
Hydrogen explosion
One of the materials used in the alloy used to produce fuel rods (the rods that hold fuel pellets) inside the reactor is zirconium. It is very good for this purpose because it doesn´t absorb neutrons and at the same time it provides strength.
The problem is that in case of overheating, the zirconium reacts with water and produces hydrogen, which is explosive. If the hydrogen leaks out of the reactor and mixes with oxygen , an explosion can occur (that is what happened at Fukushima).